chanel e coronavirus | Atomic structure of the open SARS chanel e coronavirus The calcium channel activity of E is associated with the inflammatory responses of COVID-19. Using solid-state NMR (ssNMR) spectroscopy, we have determined the open-state . adidas X 18.1. Which of you is like me? Which one of you misses the legendary and famous F50 line from adidas? The idea at the time was to completely change the look of adidas soccer with the introductions of the adidas X, ACE, and Messi boots.As much as both the X and Messi were marketed as better versions of the F50 .
0 · The SARS
1 · The Coronavirus E Protein: Assembly and Beyond
2 · Targeting a coronavirus ion channel could yield new Covid
3 · SARS
4 · Discovery of SARS
5 · Coronavirus envelope protein activates TMED10
6 · Coronavirus envelope protein
7 · Atomic structure of the open SARS
$80.00
The SARS
how to check if gucci bag is authentic
MIT chemists discovered the structure of the “open” state of the coronavirus E channel, which contributes to the inflammation seen in cells of Covid-19 patients. The structure .The natural product proanthocyanidins are widely used as cosmetic, suggesting a potential of proanthocyanidins as disinfectant for external use. This study further demonstrates that 2-E . The E protein of severe coronaviruses contains an SS/DS motif, which is not present in milder strains and facilitates interaction with TMED10. This interaction enhances . The calcium channel activity of E is associated with the inflammatory responses of COVID-19. Using solid-state NMR (ssNMR) spectroscopy, we have determined the open-state .
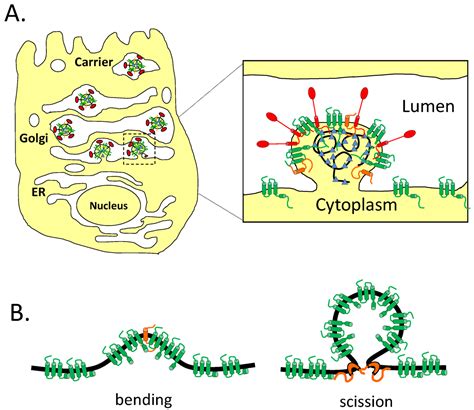
The envelope (E) protein is the smallest and least well-characterized of the four major structural proteins found in coronavirus virions. It is an integral membrane protein less than 110 amino acid residues long; in SARS-CoV-2, the causative agent of Covid-19, the E protein is 75 residues long. Although it is not necessarily essential for viral replication, absence of the E protein may produc.
A new class of 2-E channel inhibitors exhibiting antiviral activity both in vitro and in vivo were identified in the current study. Our results suggest that the physiological role of the E protein is the release of Ca 2+ from the ER, and that the distinct Ca 2+ activation site may serve as a promising target for .The coronavirus E protein is a small membrane protein that has an important role in the assembly of virions. Recent studies have indicated that the E protein has functions during infection . Taken together, three premises motivated us to examine if SARS-CoV-2 E protein is an ion channel: (i) E proteins from several coronaviruses were shown to posses channel .
In November 2021, a new variant of SARS-CoV-2, the Omicron variant, emerged and spread in more than 26 countries around the world. The Omicron variant has higher .
MIT chemists discovered the structure of the “open” state of the coronavirus E channel, which contributes to the inflammation seen in cells of Covid-19 patients. The structure could guide researchers in developing antiviral drugs that .The natural product proanthocyanidins are widely used as cosmetic, suggesting a potential of proanthocyanidins as disinfectant for external use. This study further demonstrates that 2-E channel is an effective antiviral drug target and provides a potential antiviral candidate against SARS-CoV-2. Keywords: SARS-CoV-2, envelope protein (2-E . The E protein of severe coronaviruses contains an SS/DS motif, which is not present in milder strains and facilitates interaction with TMED10. This interaction enhances TMED10-oligomerization,.
The Coronavirus E Protein: Assembly and Beyond
The calcium channel activity of E is associated with the inflammatory responses of COVID-19. Using solid-state NMR (ssNMR) spectroscopy, we have determined the open-state structure of E’s transmembrane domain (ETM) in lipid bilayers.The envelope (E) protein is the smallest and least well-characterized of the four major structural proteins found in coronavirus virions. [2] [3] [4] It is an integral membrane protein less than 110 amino acid residues long; [2] in SARS-CoV-2, the causative agent of Covid-19, the E protein is 75 residues long. [5] A new class of 2-E channel inhibitors exhibiting antiviral activity both in vitro and in vivo were identified in the current study. Our results suggest that the physiological role of the E protein is the release of Ca 2+ from the ER, and that the distinct Ca 2+ activation site may serve as a promising target for channel blockers, potentially inhibiting the infectious cycle of coronaviruses.
The coronavirus E protein is a small membrane protein that has an important role in the assembly of virions. Recent studies have indicated that the E protein has functions during infection beyond assembly, including in virus egress and in the host stress response.
Taken together, three premises motivated us to examine if SARS-CoV-2 E protein is an ion channel: (i) E proteins from several coronaviruses were shown to posses channel functionality [13, 15, 16, 18]; (ii) Ion channels are excellent targets for pharmaceutical point inhibition; and (iii) Coronavirus E proteins are important for viral virulence . In November 2021, a new variant of SARS-CoV-2, the Omicron variant, emerged and spread in more than 26 countries around the world. The Omicron variant has higher transmissibility and incidence. Xia et al. analyzed the effect of the T9I mutation of 2-E channel in the protein sequence of this variant.
MIT chemists discovered the structure of the “open” state of the coronavirus E channel, which contributes to the inflammation seen in cells of Covid-19 patients. The structure could guide researchers in developing antiviral drugs that .The natural product proanthocyanidins are widely used as cosmetic, suggesting a potential of proanthocyanidins as disinfectant for external use. This study further demonstrates that 2-E channel is an effective antiviral drug target and provides a potential antiviral candidate against SARS-CoV-2. Keywords: SARS-CoV-2, envelope protein (2-E . The E protein of severe coronaviruses contains an SS/DS motif, which is not present in milder strains and facilitates interaction with TMED10. This interaction enhances TMED10-oligomerization,. The calcium channel activity of E is associated with the inflammatory responses of COVID-19. Using solid-state NMR (ssNMR) spectroscopy, we have determined the open-state structure of E’s transmembrane domain (ETM) in lipid bilayers.
The envelope (E) protein is the smallest and least well-characterized of the four major structural proteins found in coronavirus virions. [2] [3] [4] It is an integral membrane protein less than 110 amino acid residues long; [2] in SARS-CoV-2, the causative agent of Covid-19, the E protein is 75 residues long. [5] A new class of 2-E channel inhibitors exhibiting antiviral activity both in vitro and in vivo were identified in the current study.
Our results suggest that the physiological role of the E protein is the release of Ca 2+ from the ER, and that the distinct Ca 2+ activation site may serve as a promising target for channel blockers, potentially inhibiting the infectious cycle of coronaviruses.The coronavirus E protein is a small membrane protein that has an important role in the assembly of virions. Recent studies have indicated that the E protein has functions during infection beyond assembly, including in virus egress and in the host stress response. Taken together, three premises motivated us to examine if SARS-CoV-2 E protein is an ion channel: (i) E proteins from several coronaviruses were shown to posses channel functionality [13, 15, 16, 18]; (ii) Ion channels are excellent targets for pharmaceutical point inhibition; and (iii) Coronavirus E proteins are important for viral virulence .
1-48 of 882 results for "Women's adidas Leggings" Results. Price and other details may vary based on product size and color. Overall Pick. +14. adidas. Women's Essentials 3-Stripes Leggings. 5,711. 100+ bought in past month. $1841. List: $40.00. FREE delivery Thu, Jun 6 on $35 of items shipped by Amazon. +10. adidas.
chanel e coronavirus|Atomic structure of the open SARS